Exploring the Benefits of Recombinant Protein Purification
Feb 20th 2023
Production of recombinant protein using the microbial system has helped rapidly revolutionize biochemistry. We are past the era where large amounts of plant and animal tissues or large volumes of biological fluids were required to purify small amounts of a given protein. Anytime you want to carry out a project that requires purified protein, you should go for recombinant proteins, which are easy to express and purify. The ability to efficiently produce and purify recombinant protein in large quantities ensures easy biochemical characterization, commercial goods development, and industrial use.
The steps required for expressing recombinant protein are simple. Additionally, with improved technology, the process is faster and easier. Nevertheless, there are possible impurities that are likely to accumulate during the process, so your protein must undergo a thorough purification.
This article will explore various benefits of the purification of recombinant protein.
What are Recombinant Proteins?
Before proceeding, let’s begin by getting a quick idea of what are recombinant proteins. Besides, recombinant protein productionhappens through the transfection of foreign genes into a host cell. There are various recombinant protein uses, such as producing pharmaceutical products, producing antibodies and recombinant enzymes for the treatment of diseases, protein-based polymers for drug delivery, and making protein scaffolds for tissue engineering, among other multiple uses. Researchers also use recombinant protein in their projects.
The Emergence of Recombinant Protein
Peter Lobban first proposed the idea of recombinant protein. He was the first to express and replicate the recombinant DNA in 1973. The recombinant DNA is produced through fusing sequences that may not be found in an organism. Nevertheless, you can change and re-insert a modified DNA as most organisms have similar DNA structures.
The altered DNA is then introduced to the host cell, after which it is replicated without any expression, or it can be transcribed and translated, leading to recombinant protein creation. In most cases, more sequences, such as translation initiation signals or promoters, may be needed to catalyze the expression of recombinant DNA. The expression of Recombinant protein has, however, evolved and improved tremendously. Recombinant protein technology is also improving as more and more efficient methods keep on emerging.
Expression of Recombinant Protein
The methods used to produce recombinant protein involve transfecting specific cells with a DNA vector with a template of recombinant DNA. The cell with the template is then cultured to help transcribe and translate the protein required.
The cells can be broken or lyzed to release the created protein, which is later purified. The systems used in expressing recombinant DNA include eukaryotic and prokaryotic systems. The choice of the system is determined by the type of protein you need, the functionality activity, and the amount required.
Here are the Common Host Systems Used in Recombinant Protein Expression
There are several recombinant protein expression systems commonly used;
1. Mammalian Systems
Mammalian systems are used for various purposes, such as performing structural analysis, protein interaction, custom antibody production, functional assays, and the production of viruses. The main advantage of using this system is that you will get stable production of protein and the system is also transient.
Additionally, the system can be used for the rapid production of large quantities of proteins. Nevertheless, there are some disadvantages associated with the use of mammalian systems. For example, in this system, the cell culture conditions are more demanding, and also, there might be a high need for large yields, such as liters, if there is a suspension of cultures.
2. Insect System
The insect system is commonly used for expressing protein complexes and intracellular proteins, performing structural analysis, producing viruses, and performing functional assays, among other applications. The expression process in the insect system is similar to the process used in expressing the protein in mammalian systems. Nevertheless, the cell culture condition may be more demanding in an insect system than in prokaryotic cell culturing. Additionally, the production of recombinant vectors may take a long time when using an insect system.
3. Yeast System
Like mammalian and insect systems, the yeast system is also used to perform functional assay and structural analysis. Additionally, it is used to analyze generational antibodies and study protein interactions. A yeast system can be applied in processing eukaryotic proteins through fermentation. The system has simple requirements. Nevertheless, to produce high-yield proteins, fermentation is essential, and growth conditions must be optimized.
4. Bacterial System
Bacterial System is the commonly used host due to low cost, scalability, and simple media requirements. It can also be used in functional and structural assay analysis. The most commonly used bacterial system host is E.coli because it has fast growth kinetics, you can quickly achieve high cell density cultures, and recombinant DNA is easy and fast. However, this system may need to express some mammalian proteins more efficiently.
Example of Recombinant
- Human insulin used in the treatment of diabetes
- Human growth factors used to treat hormone deficiency
- factor VIII is commonly used to treat hemophilia
- therapeutic monoclonal antibodies used in treating cancer and viral infections
- Research reagents include inhibitors, ELISA and fluorescence assays, protein folding, and small molecules.
Before the invention of molecular biology and the expression of recombinant proteins technology, these proteins were obtained directly from animal sources. The technology led to the development of entirely artificial recombinant proteins, such as recombinant antibodies for diagnostic and therapeutic application and proteins for vaccine production.

Protein Purification
The production of recombinant proteins, primarily through bacterial hosts and vectors, is a well-advanced and defined technology. Nevertheless, isolating it into an active form is a big problem. Therefore, purification or recombinant protein is a vital process in biological research. To study a protein's particular structure and function, you must use pure recombinant protein. This means you must isolate and purify it before use. Protein purification, therefore, is a process that involves the solution of required proteins from a concentrated form of other unwanted tissues, cells, or proteins.
The protein purification technology primarily uses similarities and differences between various recombinant proteins. For example, non-proteinaceous materials such as tissues and cells can be eliminated based on similarity. On the other hand, the required protein can be isolated based on the differences between the proteins.
Protein tags are vital and convenient tools that help streamline protein purification, enhancing the solubility of required recombinant protein and providing an easy method to track proteins during the recombinant protein expression and purification process. Further, protein tags are essential for tracking proteins and processes using microscopic or indirect methods such as Immunoprecipitation, Western blot, or immunostaining.
Common Purification Methods Used in Recombinant Protein
1. Extraction
This purification technique isolates the protein by breaking cells or tissues from the cells of the host systems. If the organism produces no protein, the first vital step is ensuring the cell is disordered where proteins are located. You can use various methods such as repeated freezing, homogenization via high grinding or pressure, thawing, sonication, or permeabilization by enzymes or detergents. Additionally, the extraction method produces proteases during cell lysis, which enables the beginning of the protein's digestion in the solution.
If there is a reaction between protein and proteolysis, you should quicken the purification and ensure the extract is cool to manage the process of digestion. Alternatively, you can use protease inhibitors. The extraction method used in the purification process depends on host cells and protein fragility. Soluble protein can also remain solvent and be separated from the cell membrane via centrifugation.
2. Chromatography
One of the most popular purifying techniques is chromatography. This is a result of its many benefits compared to other purifying techniques. For instance, high-resolution efficiency in chromatography enables the isolation of complex and abrasive mixtures, including those containing molecules. Also, chromatography is a convenient method for trapping diluted molecules in a highly diluted solution.
The main idea behind the chromatography technique is to separate larger recombinant protein pools into smaller ones that contain a lot of the target protein. The method uses specific and essential equipment. Additionally, the purification method adopts a series of steps. For example, the protein solution flows through columns occupied with necessary components as the first important step in this procedure. The column materials allow the numerous types of protein to interact in various ways.
Here, a particular chromatography strategy is applied in recombinant protein purification: metal binding size, affinity chromatography, and Immunoaffinity chromatography.
Precipitation and Differential Solubilisation
The differential Solubilisation and precipitation procedures work best to remove significant amounts of proteins. One of the essential reagents utilized in this approach is ammonium sulfate. It helps precipitate proteins by adding a considerable amount of ammonium sulfate and then collecting the different precipitate protein portions.
Initially, the recombinant proteins enter the precipitation process, where the host cell makes certain that the target proteins are well-concentrated before moving on to additional processes with pre-destined purification columns. The recombinant protein can precipitate naturally as an inclusion body due to a variety of variables and effects in the expression host system. Precipitation and Differential Solubilisation is one of the most affordable and cost-effective methods for recombinant protein purification and is ideal for purifying large proteins.
Ultracentrifugation
This purifying technique, called ultracentrifugation, separates a combination of specific molecules with varying densities and masses using centrifugal force. The object's acceleration affects every particle of the protein mixture to exert an outward pressure equal to its mass as the object is spun at high speed. The targeted particles are then transmitted in the liquid using a centrifugal force.
The particles can flow through the fluid because of an opposite strength applied to them. Compared to other less massive molecules, heavy and huge particles will move outward when you spin the sample in a centrifuge.
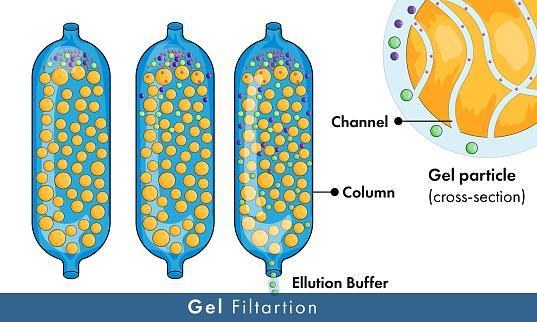
Gel Filtration
Gel Filtration is a convenient and accessible purification method for separating components of different sizes. It is part of the chromatography method. The solution is first packed in columns to help perform the purification process easily and fast. The arrangement led to what is known as a packed bed formation. The particles and molecules have different physical and chemical stability, so the component must be packed in a porous matrix. The packed bed is moderated through a hedge that fills the particles' matrix pores and the remaining spaces.
The primary principle of this method is that the giant molecules are filtered from the pores. On the other hand, the small molecules are allowed to go through the interior of the pores either wholly or partly. For example, it's easy to filter recombinant proteins using gel filtration since their particles are smaller than others.
Here are the Benefits of Purification of Recombinant Protein
- High-Quality Protein; Purification process ensures that you get high-quality recombinant proteins with zero contaminants or unwanted materials such as tissues or cells. This means that the produced recombinant proteins, when used for research, medicine, and vaccine productions, will give better quality outcomes.
- Easy to Get Specific Protein; the produced proteins may contain other unwanted proteins from the host system. Therefore, purification helps to ensure you get specific proteins by separating the target proteins from other proteins. This means that, with various purification methods, it's possible now to get the particular pure protein quickly.
- Purification Helps in Protein Characterization; Protein Characterization is a process that uses experimental methods to detect and isolate proteins. It helps in the definition of the structure and interactions of the target protein. Protein purification is a vital process in the protein characterization process.
- Through recombinant protein purification, it is possible to assess and establish the various biochemicals, such as signaling capacity and enzymatic activities, that reside in some unique proteins.
- Pure recombinant proteins are valuable in producing biochemical reagents such as hormones or growth factors, DNA polymerases, proteases, ligases, antibodies, or phosphatases.
- Purified proteins enable studying and understanding their enzymology and affinity for specific substrates. Such knowledge lets you know how various biological methods can work as catalysts in metabolic processes.
- Purified recombinant proteins make using ELISA kits and fluorescence assays easy.
Wrapping Up
Recombinant protein purification is a primary concern in the biochemist field. Due to continuous and thorough research, the recombinant protein expression and purification process have significantly evolved, producing large and high-quality proteins. Additionally, the various purification methods, which have been improved considerably, have ensured the yield of pure and quality-specific proteins. If you don’t have the necessary equipment, knowledge, and skills, you can sort for affordable recombinant protein expression and purification services from your nearest laboratory. Before embarking on any research project that requires recombinant proteins, ensure that you can access pure and quality protein for better results.

