An Easy Guide To Antibody Production
Apr 25th 2022
An Easy Guide To Antibody Production
Animal immune systems can make antibodies that attach to detect substances of interest in several investigative and analytical applications. No other contemporary method allows researchers to develop and produce molecular recognition tools with excellent specificity. Nearly all medical and cell biology researchers use Antibody technology to do molecular analysis. The amount to which scientists are concerned with antibody synthesis varies depending on their research demands. What Are Antibodies?
Image Source: https://pixabay.com/photos/virus-pathogen-antibody-antibodies-5741636
Antibodies are components of a host defensive mechanism generated in response to foreign molecules (antigens) entering the host. The host defense system detects them molecularly, leading to the formation of immunoglobulin that can bind to the particular antigen.
The Formation of Antibodies
To understand the formation of antibodies, we should ask ourselves, does protein make antibody? The B-lymphocyte protein makes antibodies that migrate through the blood and lymph, binding to their target cell and removing it from circulation. Animal immune systems’ capacity to produce antibodies that attach to antigens may be used to make probes for detecting substances of interest in many scientific and analytical applications.
Now you know how antibodies are formed in animal immune systems. No other contemporary method allows researchers to develop and produce molecular recognition systems with excellent specificity.
Antibodies are especially well suited to advancement as probes due to several critical characteristics, including their high specificity. Procedures for antibody synthesis and protein purification services were established in the 70s and 80s.
Structure and characteristics of antibody isotypes
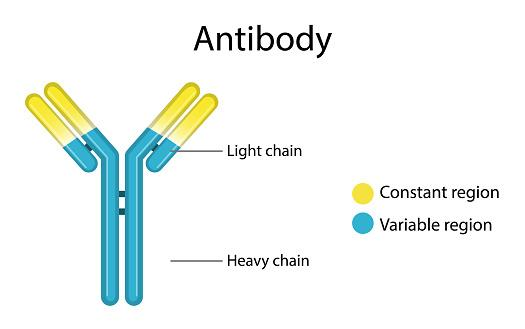
Image Source: https://www.istockphoto.com/vector/antibody-structure-diagram-on-white-background-gm1268952516-372538851
All human antibodies are divided into five isotypes based on the unique H chains (immunoglobulins M, D, G, A, and E). Each of these antibody isotypes has special antibody validation features and functions, thanks to these isotopes. Identifying the isotype class and subclasses is essential in custom antibody production.
IgG
All human immunoglobulins are 70-75 percent IgG antibodies in the blood (plasma) (antibodies). Leukocytes and macrophages use IgG to discover antigen-antibody interactions and detoxify hazardous substances. The umbilical cord transmits IgG in the womb, protecting the unborn child until their immune system is completely grown.
IgM
Roughly 10 percent of the total of all immunoglobulins in the human body are made up of IgM, which passes through the circulation. IgM is composed of a pentameric structure made up of five Y-shaped components. Initially, B cells produce IgM due to pathogenic infection/antigen invasion. Despite its lower tolerance for antigens, IgM’s pentameric/hexameric structure makes it more receptive to antigens than IgG.
IgA
IgA is present in several body fluids, making up 10-15 percent of a person’s immune system cells. There are two kinds of IgA dimers. IgA in breast milk protects newborns’ gastrointestinal systems against infection.
IgD
Human immunoglobulins only include a minuscule amount of IgE, less than 1% of all immunoglobulins. Its original purpose was to keep parasites at bay. The antibody IgE is most likely to blame when allergic responses occur, even in regions free of parasite infestation. Only a sliver of the world’s immunoglobulins is human immunoglobulins (IgD).
Custom Antibody Production
The adaptable response is the mechanism of antibody production by the immune system to fight infections that have infiltrated the host. The offending bacterium, viruses, or some other organism, generally referred to as antigens, is recognized by the antibody with particular lock and essential identification. Antibody production procedures and antibody sequencing services may be used to create polyclonal and monoclonal antibodies and other biologics.
Proteins are called antibodies, which are pleated polypeptides or threads of amino acids that include complimentary antigen sequences that uniquely identify a binding site on a particular antigen. Antibodies are produced by which cells? The B-cells of the host defense system.
How to develop antibodies?

Image Source: https://pixabay.com/photos/covid-19-coronavirus-dystopia-4987797/
Now that you know how to make antibodies in the human body, let’s review how to develop antibodies outside the body and their uses in the research community. The specificity of antibody-antigen recognition has a wide range of applications in biotechnology and the construction of sensors. These antibodies are often attached to sensor surfaces to detect antigens.
They may be employed in conjunction with either a nanoparticle or another labeled detection platform to enhance their detection capabilities. Purified antibodies with high specificity for a specific antigen are manufactured in America. An example of a renowned expert in custom protein production services is the Elisa Kits Manufacturers, which has optimized over 2000 protein-specific Elisa Kits.
The use of a protein expression system enables the investigation of gene regulation and peptide anatomy and physiology. Additionally, the applications of recombinant protein expression systems may be pretty diverse, ranging from in vivo function inquiry to large-scale synthesis for biotherapeutic drug development and structural investigations. Success in protein expression requires selecting the most appropriate recombinant expression system.
When selecting an expression system like the e.coli expression systems, consider protein binding, efficiency, separation rate, and output. For your research requirements, we provide a comprehensive range of quality mammalian, insect, and yeast protein expression systems and microbial, microalgae, and biofilm protein expression and purification services from well-known companies such as Gibco and Invitrogen. These two companies also excel in how to analyze Elisa data because the companies use advanced expression systems. An example of an advanced expression system is the e.coli expression systems.
Depending on how they are created and collected, antibodies may be divided into polyclonal and monoclonal antibodies. Polyclonal antibodies can detect several epitopes or places on an antigen, making them useful in immunological research. A variety of B cell lineages are responsible for their development. Antibodies that identify just one epitope on an antigen are known as monoclonal antibodies, and they are generated from a single B-cell line. In other sectors, such as custom protein expression and protein expression services, several vendors like ProteoGenix have found success.
Suppose an antibody is required against a particular antigen that is not widely accessible from a provider. In that case, it is possible to have antibodies manufactured via bespoke antibody synthesis. A sample of antigen that has been 90 percent purified is all that is required to have a bespoke antibody produced and harvested against that antigen. Alternatively, if a sample of the antigen cannot be obtained, it is possible to have bespoke peptides or proteins manufactured through custom protein synthesis and antibodies generated against them.
Production of Polyclonal Antibodies
The polyclonal antibody production service shall continue to be an essential research endeavor. Rabbits will be one of the principal species employed in polyclonal antibody synthesis, as they have been for many years. Vaccination schedules and procedures for immunizing rabbits and producing polyclonal antibodies shall vary tremendously, depending on the immunomodulator, the adjuvant, and the ultimate use for which the antibody is intended.
It is important to note that the selection of adjuvant and vaccination schedules is a critical element of the polyclonal antibody manufacturing operation that is often disregarded as researchers rely on conventional published procedures that may or may not suit their requirements. The process regarding how to make polyclonal antibodies usually takes 3-5 steps depending on the purity of the available materials.
The large histologic lesions caused by FCA, although still the most efficient adjuvant for polyclonal antibody generation, will continue to spur the discovery of alternative adjuvants that induce minor tissue damage and less potential discomfort and anguish.
The Process of Polyclonal Antibody Production:
- Design of peptides through custom peptide synthesis
- Animal vaccination
- Serum collection
- Titer analysis
- The ultimate antibody purification process
If the client provides an antigen sample that is 90 percent pure, the first two processes are not required to be completed, especially peptide synthesis. It takes approximately 80 days to manufacture custom polyclonal antibodies from bunnies, piggies, poultry, goat, and rodents, with most antibodies made from chicken. Because they react to most antigens, bunnies are utilized in 95 percent of all experiments. They have a higher-yielding immune function that manufactures antibodies quickly.
In most cases, it takes 70 to 120 days based on the procedure that is being employed. Antiserum produced by rats and mice is only beneficial in tiny quantities. Goats have the most significant production, but they react to antigens more slowly (minimum 120 days). Chicken is only utilized if the rabbits do not respond or produce antibodies and the chicken is not available. Piggy antibodies have a lower context in drosophila data analysis and may be used as secondary antibodies in certain situations.
Production of monoclonal antibodies
Polyclonal antibodies are not always the best choice for some tests because they lack the specificity and affinity that custom monoclonal antibodies provide. To achieve such remarkable precision, every one of the antibodies should attach with complex formation to a single epitope, which is challenging. Monoclonal antibodies, which have high specificity, are available (mAbs). In contrast to polyclonal antibodies generated in live animals, Monoclonal antibodies are created in vitro using epithelial procedures rather than in animals.
The manufacture of custom monoclonal antibodies takes around six months from start to finish.
The following are custom monoclonal antibody production steps:
- Preparation of antigens
- Development and immunizations of hybridomas
- Development and fusions of hybridomas
- Subcloning
- Monoclonal manufacturing and purifying
Immunoglobulins (mAbs) are created by repeatedly injecting a particular antigen into an animal, most often a mouse. After that, B cells from the spleen of the vaccinated animal are eliminated from the body. Because normal B cells have a limited ability to be ever-increasing.
A monoclonal antibody specific to the hybridomas may then be evaluated for development in culture for an infinite period (mAb). Cells that generate the required antibody (mAb) are grown in tissue culture; the culture media is withdrawn often, while antibodies are isolated from the medium. This is a lengthy and expensive protocol that will take months to complete. It may take weeks of culture and many liters of the medium to develop enough antibodies. The cost of mAbs is out of reach for most people, so only well-funded research scientists and organizations use it for custom protein production and protein production services.
Polyclonal Antisera in Medicine

Image Source: https://pixabay.com/photos/corona-vaccine-medical-staff-6335210/
Many clinical diagnostics employ polyclonal antisera to assess if a patient generates antibodies against a particular infection. These assays are excellent diagnostic tools, but they have limits since they are indirect methods of detecting pathogen presence. Polyclonal tests may occasionally provide false-positive results, indicating an antigen’s existence or nonexistence. Antibody-based testing may give false-negative results when the test misses an antibody present.
The antibody test accuracy is measured by sensitivity and specificity. A positive diagnosis is likely to happen when the patient is infected. A high sensitivity test has a low chance of being a false negative. Cross-reactivity occurs when epitopes from one pathogen are identical to another.
Consequently, antibody-based diagnostics are often employed solely as screening tests, with further tests to confirm positive findings. For example, antibodies to hepatitis C antigens may screen blood samples from patients suspected of possessing the virus. If the patient has hepatitis C, the antibodies will attach to the antigens, resulting in a positive test. Antisera may be used to identify bacteria in clinical and food industry contexts and execute various precipitation processes to detect serum proteins or pathogens effectively. However, polyclonal antibodies are not utilized in custom gene synthesis.
Monoclonal Antibodies in Medicine
Because most monoclonal antibody sequencing techniques involve mouse cells, humanized monoclonal antibodies are required for clinical application. Humans cannot be repeatedly injected with mouse antibodies since the immune response will detect them as alien and produce neutralizing antibodies. According to gene synthesis companies, the antibody in mouse B cells may be genetically modified to reduce this issue. The mouse light and heavy chain genes’ variable regions are covalently attached to constant human parts and subsequently transported into a host cell. Just the antigen-binding site of a mAb is mouse-derived.
Humanized mAbs are being used effectively to treat cancer. For example, Herceptin, a humanized monoclonal antibody produced by peptide synthesis companies, has helped treat certain breast cancers. There have been several early humanized mAb studies for treating infectious disorders, but none are now in use. In other circumstances, mAbs are overly selective, recognizing just specific serovars of a virus. Using a mix of mAbs that target distinct pathogen strains may help. The high cost of mAb manufacture has also precluded mAbs from being used to treat microbial illnesses.
Using GM plants to manufacture antibodies is one potential method for low-cost mAbs (or plantibodies). Rather than using pricey and technically challenging tissue culture cells, this approach results in antibodies produced by plant cells. Patients might get antibodies by eating plants rather than extracting and injecting them in rare circumstances. For example, in 2013, scientists replicated antibody genetic material into plants which might neutralize a bacterial toxin that causes severe gastrointestinal sickness.
In Summary
When it is essential to generate an antibody vs. an uncommon or distinctive antigen, custom antibody manufacturing is a crucial tool at your disposal. The process, which is particularly useful for monoclonal antibody production, is also beneficial to others in the scientific community because, once a hybridoma cell line has been established to produce an antibody, it is practicable to maintain that cell line for use in other experiments in the coming years.

